[Written by ChatGPT. Feature image from Avea]
The 2023 article “Hallmarks of aging: An expanding universe“ by Carlos López-Otín and colleagues updates the original 2013 framework of aging by adding new hallmarks and refining our understanding of the aging process. Here’s a summary of the key points:
Core Concept
Aging is a complex biological process driven by multiple interconnected mechanisms. The authors originally identified nine hallmarks of aging but have now expanded the framework to include new hallmarks based on advances in the field.
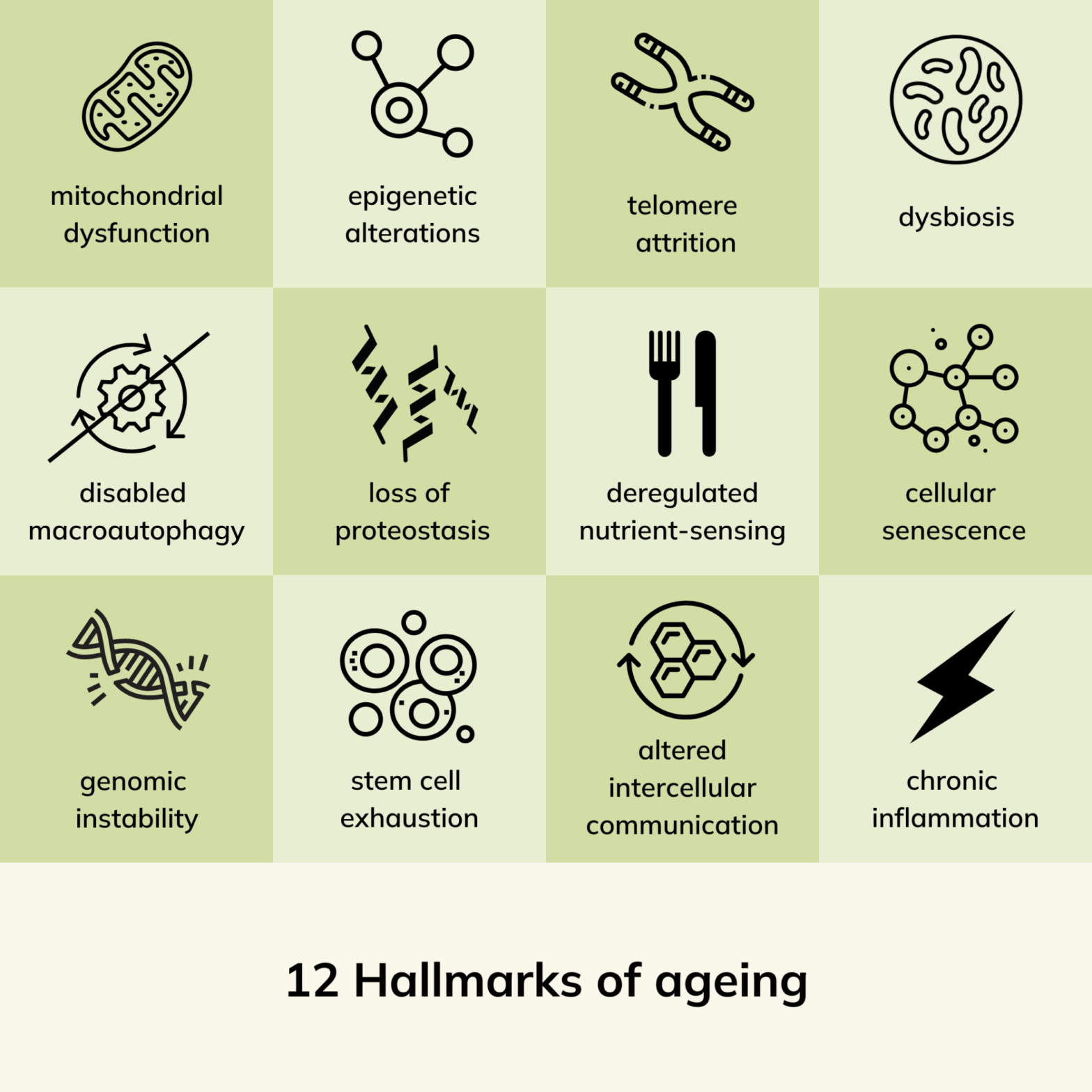
The Original Nine Hallmarks (2013)
- Genomic Instability
Accumulation of DNA damage and mutations. - Telomere Attrition
Progressive shortening of chromosome ends. - Epigenetic Alterations
Changes in gene expression regulation. - Loss of Proteostasis
Decline in protein maintenance systems. - Deregulated Nutrient Sensing
Imbalances in metabolic pathways like mTOR and AMPK. - Mitochondrial Dysfunction
Reduced mitochondrial efficiency and increased reactive oxygen species. - Cellular Senescence
Accumulation of non-dividing but metabolically active cells. - Stem Cell Exhaustion
Decline in regenerative capacity. - Altered Intercellular Communication
Chronic inflammation and disrupted signaling.
Newly Added Hallmarks (2023)
- Disabled Macroautophagy
Impaired cellular cleanup mechanisms, such as autophagy, which remove damaged organelles and proteins. - Chronic Inflammation
Persistent, low-grade inflammation (inflammaging) contributes to tissue damage and systemic aging. - Dysbiosis
Age-associated imbalances in the gut microbiome disrupt immune function, metabolism, and overall health.
Key Insights from the Update
- Interconnectedness:
The hallmarks are not isolated but form a network of interdependent processes. For example, genomic instability can exacerbate inflammation, and mitochondrial dysfunction can impair autophagy. - Progressive Understanding:
The addition of the three new hallmarks highlights the dynamic and expanding nature of aging research as new discoveries emerge. - Intervention Strategies:
Addressing these hallmarks collectively rather than in isolation could lead to more effective therapies. Examples include senolytics (removing senescent cells), microbiome modulation, and autophagy enhancers.
Clinical Implications
- Aging interventions could delay or prevent age-related diseases by targeting specific hallmarks.
- A systems biology approach is necessary to understand and tackle the complexity of aging.
Clinical and Individual Actions Addressing Hallmarks of Aging
Addressing the hallmarks of aging requires both clinical approaches (medical interventions) and individual actions (lifestyle and behavioral changes). Here’s a breakdown for each hallmark:
1. Genomic Instability
Clinical Approaches:
- Gene Therapy: Repair DNA damage using CRISPR or other gene-editing tools.
- DNA Repair Enhancers: Drugs targeting DNA repair pathways (e.g., PARP inhibitors for some cancers).
- Antioxidants: Mitochondria-targeted antioxidants to reduce DNA damage from reactive oxygen species (e.g., MitoQ).
Individual Actions:
- Minimize exposure to environmental mutagens (e.g., UV radiation, tobacco, and pollution).
- Consume antioxidant-rich foods (berries, leafy greens, nuts).
- Prioritize sleep, as it aids in DNA repair processes.
2. Telomere Attrition
Clinical Approaches:
- Telomerase Activators: Drugs or molecules like TA-65 (early-stage research) aim to extend telomeres.
- Cell Therapy: Use of stem cells with longer telomeres.
Individual Actions:
- Manage stress through meditation, yoga, or mindfulness, as chronic stress shortens telomeres.
- Regular physical activity, particularly aerobic exercise.
- Balanced diet with anti-inflammatory properties.
3. Epigenetic Alterations
Clinical Approaches:
- Epigenetic Drugs: Modulate DNA methylation or histone acetylation (e.g., HDAC inhibitors in cancer therapy).
- Reprogramming Technologies: Cellular reprogramming (e.g., Yamanaka factors) to reverse epigenetic aging.
Individual Actions:
- Maintain a nutrient-dense diet with epigenetic-impacting nutrients (e.g., folate, B12, choline, and polyphenols).
- Avoid smoking and excessive alcohol consumption.
- Engage in regular physical activity, which promotes healthy epigenetic changes.
4. Loss of Proteostasis
Clinical Approaches:
- Proteostasis Modulators: Drugs enhancing autophagy and proteasome activity (e.g., rapamycin, spermidine).
- Heat Shock Protein Inducers: Boost cellular defenses against protein aggregation.
Individual Actions:
- Periodic fasting or time-restricted eating to stimulate autophagy.
- High-quality protein intake to maintain muscle mass and repair mechanisms.
- Avoid exposure to high temperatures or toxins that cause protein misfolding.
5. Deregulated Nutrient Sensing
Clinical Approaches:
- mTOR Inhibitors: Drugs like rapamycin mimic calorie restriction benefits.
- Caloric Restriction Mimetics (CRM): Resveratrol, metformin, and NAD+ boosters.
Individual Actions:
- Practice intermittent fasting or caloric restriction.
- Reduce consumption of refined sugars and processed foods.
- Include foods that enhance sirtuin activity (e.g., berries, olive oil).
6. Mitochondrial Dysfunction
Clinical Approaches:
- NAD+ Precursors: Supplementation with NAD+ boosters (e.g., NMN, NR).
- Mitochondrial Biogenesis Activators: PQQ and resveratrol.
- Mitochondria-Targeted Therapies: Antioxidants like MitoQ or therapies like mitochondrial replacement.
Individual Actions:
- Regular aerobic exercise to improve mitochondrial function.
- A diet rich in omega-3 fatty acids and CoQ10.
- Cold exposure (e.g., cold showers, cryotherapy) to enhance mitochondrial efficiency.
7. Cellular Senescence
Clinical Approaches:
- Senolytics: Drugs that selectively eliminate senescent cells (e.g., dasatinib + quercetin).
- SASP Modulators: Inhibit inflammatory signals from senescent cells.
Individual Actions:
- Regular exercise to reduce senescence accumulation.
- Adopt an anti-inflammatory diet (rich in fruits, vegetables, and omega-3s).
- Practice stress management, as stress promotes senescence.
8. Stem Cell Exhaustion
Clinical Approaches:
- Stem Cell Therapies: Transplantation of healthy stem cells or enhancing existing stem cell function.
- Stem Cell Rejuvenation: Use of reprogramming factors or drugs.
Individual Actions:
- Adequate sleep to support stem cell function.
- Avoid excessive alcohol or drug use, which impairs stem cell health.
- Engage in regular strength training to maintain musculoskeletal stem cells.
9. Altered Intercellular Communication
Clinical Approaches:
- Anti-Inflammatory Drugs: Target chronic inflammation (e.g., IL-1 inhibitors, NSAIDs).
- Immune Modulators: Rejuvenation of immune cells (e.g., CAR-T therapy).
Individual Actions:
- Adopt an anti-inflammatory diet (avoid trans fats, refined sugars).
- Maintain social connections to reduce systemic inflammation.
- Regular moderate exercise to enhance immune signaling.
10. Disabled Macroautophagy
Clinical Approaches:
- Autophagy Enhancers: Drugs like spermidine or rapamycin.
- Fasting-Mimicking Diets: Interventions to stimulate autophagy.
Individual Actions:
- Practice intermittent fasting or prolonged fasting (under medical supervision).
- Include foods that support autophagy (e.g., green tea, turmeric).
- Avoid overnutrition or frequent snacking.
11. Chronic Inflammation
Clinical Approaches:
- Anti-Inflammatory Therapies: Use of drugs like methotrexate or colchicine.
- Cytokine Blockers: Targeting specific inflammatory pathways.
Individual Actions:
- Regular physical activity to reduce systemic inflammation.
- Prioritize sleep and stress reduction techniques.
- Consume an anti-inflammatory diet rich in polyphenols and omega-3s.
12. Dysbiosis
Clinical Approaches:
- Probiotics/Prebiotics: Restoring gut microbiota balance.
- Fecal Microbiota Transplantation (FMT): For severe dysbiosis.
- Microbiome-Modulating Drugs: Early-stage research.
Individual Actions:
- Eat a high-fiber diet with diverse plant-based foods.
- Minimize use of unnecessary antibiotics.
- Fermented foods (e.g., yogurt, kimchi) to enhance gut health.
One thought on “Science: Hallmarks of aging”